Editor’s Note: Sign up to get this weekly column as a newsletter. We’re looking back at the strongest, smartest opinion takes of the week from CNN and other outlets.
CNN
—
In 1917, British analysts deciphered a coded message the German foreign minister sent to one of his country’s diplomats vowing to begin “unrestricted submarine warfare” and seeking to win over Mexico with a promise to “reconquer the lost territory in Texas, New Mexico and Arizona” if the US entered the world war. When it became public, the Zimmerman Telegram caused a sensation, helping propel the US into the conflict against Germany.
“Never before or since has so much turned upon the solution of a secret message,” wrote David Kahn in his classic 1967 history of secret communications, “The Codebreakers.” The Germans had taken great pains to keep their intentions confidential, and the codebreakers in London’s “Room 40” had to do a lot of work to decipher the telegram.
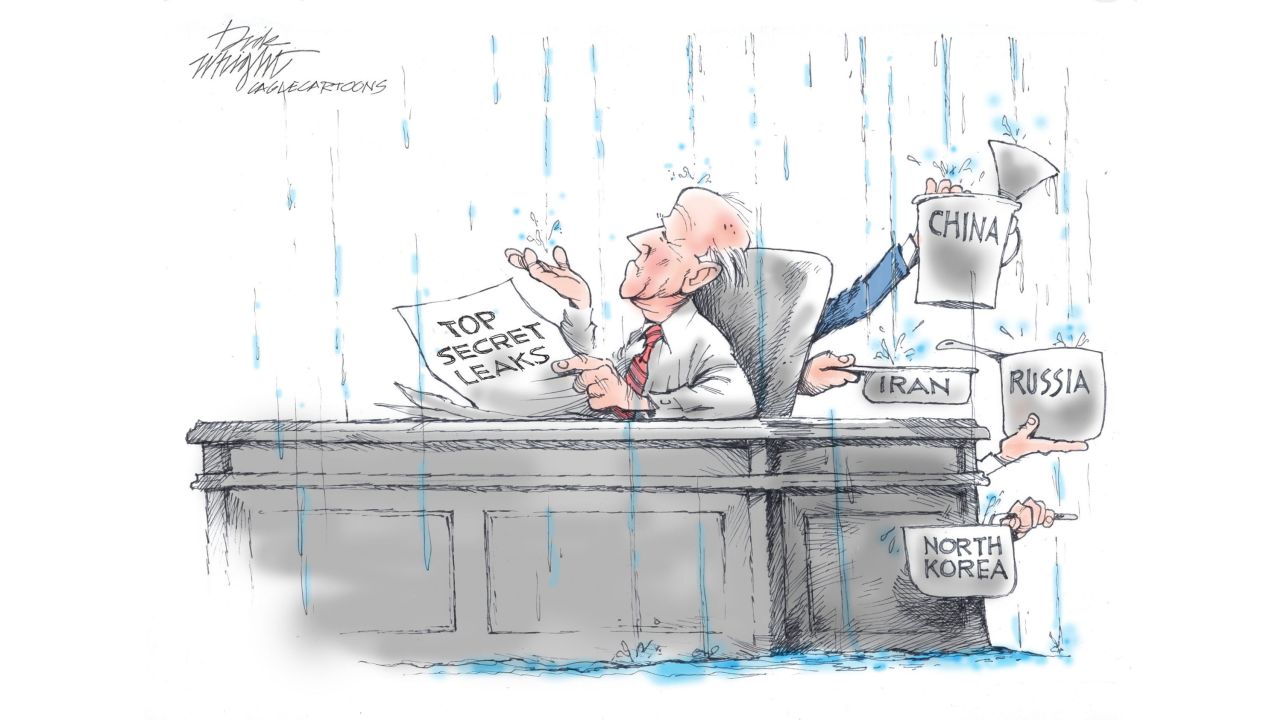
Their efforts stand in stark contrast to the ease with which secrets came tumbling out of a Pentagon intelligence network when 21-year-old Massachusetts Air National Guard cyber specialist Jack Teixeira allegedly posted hundreds of documents on a Discord chatroom known as “Thug Shaker Central.” The disclosures likely won’t start a war, but they could prove extremely damaging to the US and several of its allies, including Ukraine.
Teixeira is one of more than one million people who have Top Secret clearance. “The Pentagon has already started taking steps to limit the number of people who have access to such sensitive information,” wrote Brett Bruen, a former US diplomat and Obama administration official. “But much more can be done. … Why do so many people, especially those working short stints in government, have access to information that can shape the fate of nations and their leaders?”
Writing in the Financial Times, Kori Schake saw “some good news.”
“While specific details will be incredibly valuable to Russia and other adversaries, these are not bombshell revelations: journalists had already reported Ukrainian ammunition running low; peace talks between Moscow and Kyiv were never likely; allies have long been aware that the US eavesdrops on them; and the disparaging assessment of Ukraine’s forthcoming offensive may prove no more accurate than previous predictions were.” These will not prove as damaging as the Edward Snowden and Chelsea Manning disclosures.
But, she warned, “Technology making data ever more portable, distribution more global and communications more bespoke will make it easier to amass information and distribute it — either privately or publicly.”
In less than a week, the two Democrats expelled from the Tennessee House for their participation in a gun control protest were sent back to office by local officials.
Writing for CNN Opinion, Rep. Justin Pearson noted, “This should be a chastening moment for revanchist forces in Tennessee’s legislature and across the country. Over the long haul, the undemocratic machinations employed to oust us from office are destined to fail. Dr. Martin Luther King, Jr. once famously said that the moral arc of the universe bends toward justice. Events this week demonstrated, more than ever, that this is indeed the case…”
“Over two-thirds of Americans — including four out of 10 Republicans — support the kind of common sense gun safety laws that Rep. Jones, Rep. Johnson and I were protesting in favor of, in the wake of the senseless March 27 Covenant School massacre.”
“And yet, calls for common sense gun reform measures fall on deaf ears in our legislature where a Republican supermajority is wildly out of step with most people’s values.”
The politics of gun control have shifted, argued Democratic strategist Max Burns. The NRA’s internal struggles have weakened its influence while Democrats in office, who once feared touching the issue of guns, are increasingly speaking out. And they are making some progress in enacting new state laws, Burns noted.
“The American people decisively support Democratic proposals for addressing the scourge of gun violence. Political watchers who criticized Democrats for talking too much about abortion during the 2022 midterm elections later ate crow after that once-dreaded culture war topic topped the list of voter concerns nationally…
“Biden and the Democrats have the rare opportunity to build yet another winning coalition out of an issue once viewed as political poison.”
On Friday, the Supreme Court issued an order that temporarily ensured access to a key drug used in many medication abortions. The move gave the justices more time to consider the issue after a Texas federal judge suspended the US Food and Drug Administration’s approval of the abortion pill 23 years ago.
“If abortion opponents are successful, access to the pill — reportedly used in more than half of abortions in the United States — will be severely undercut,” wrote Michele Goodwin and Mary Ziegler.
“Beyond the dangerous precedent this sets for challenges to other important FDA-approved drugs that some political factions don’t like, the case is an alarming expression of the way right-wing activists are using junk science to bypass the will of the American public and restrict abortion…”
“There are no grounds for challenging mifepristone’s approval, especially 23 years after the fact. The drug received extensive review — more than four years — before FDA approval. Moreover, claims that mifepristone threatens the health of those who take it are unfounded. The drug has a better safety record for use than Viagra and penicillin. Notably, it was available and used for years without incident in Europe.”
In 1986, Nicholas Daniloff, the Moscow bureau chief for US News & World Report, was seized by Soviet authorities and locked up in Lefortovo prison. He was the last American journalist to be arrested in Russia before last month’s detention of Wall Street Journal correspondent Evan Gershkovich, who like Daniloff, speaks Russian fluently. Gershkovich has been charged with espionage but US officials have concluded that he was “wrongfully detained.”
As David A. Andelman noted, Daniloff’s detention in prison lasted for 13 days before he was put under house arrest and then eventually swapped for an accused Soviet spy. In a conversation with Andelman, Daniloff recalled his reaction when he was imprisoned. “I felt claustrophobic, and I felt like I wanted to get out of there immediately. Of course, there was no chance of that. The door slams, and you have all these thoughts and feelings that run through you, and then you settle down and you realize you’re going to be hanging around that cell for some time.”
Gershkovich’s family in Philadelphia received a letter, handwritten in Russian, from the reporter Friday.
“I want to say that I am not losing hope,” he noted. “I read. I exercise. And I am trying to write. Maybe, finally, I am going to write something good.”
The Amazon series “The Marvelous Mrs. Maisel” returns this month for its fifth and final season — and David Perry is here for it. The series brings back memories of visiting his grandparents Irma and Mordy in their “tiny rent-controlled Greenwich Village apartment,” an experience that helped shape his Jewish identity.
“As a Jewish historian,” Perry wrote, “I worry about the tension between preserving the memory of past hardships while not locking our entire history into a tale of oppression. The moments of peace and joy are as vital as the moments of violence. In fact, it’s the periods of peace, of success, of interfaith community, that reveal the terrible truth about the violence: it wasn’t inevitable. People could have made different choices…”
“A show like ‘The Marvelous Mrs. Maisel’ lets me revel in my personal New York Jewish heritage while also getting a little break from all the worry. It’s a warm, funny, sexy, extremely Jewish …. comedy that hits me straight in my glossy childhood memories. That isn’t to say the show isn’t also problematic — it most certainly is.”
In the latest installment of CNN Opinion’s “Little Kids, Big Questions” series, 10-year-old Ronan wonders if animals are capable of being smarter than humans. With the help of the John Templeton Foundation, which is partnering on the project, the answer came from Jane Goodall, world renowned for her work with chimpanzees.
“One of the attributes of intelligence is the ability to think and solve problems. In the early 1960s, I was told that this was unique to humans, and only we could use and make tools, only we had language and culture,” Goodall said. “But more and more research has proved that many animals are excellent at solving problems. Many use tools, and many show cultural differences. Some scientists believe that whales and dolphins are communicating with what may be a real language.”
“Although the difference between humans and other animals is simply one of degree, our intellect really is amazing. …bees can count and do math, and that just shows how much we still have to learn about animal intelligence. But humans can calculate the distance to the stars.”
Earlier this month, a Texas jury convicted Daniel Perry of murder for fatally shooting a Black Lives Matter protester in 2020. The jury deliberated for 17 hours and decided Perry’s action couldn’t be excused under the state’s “stand your ground” law. Prosecutors argued Perry had instigated the incident and they introduced into evidence messages that suggested the shooting was not a spur-of-the-moment act but a premeditated one.
On the evening of the jury verdict, Fox News host Tucker Carlson criticized the decision and told viewers he had invited Texas Gov. Greg Abbott on the show to ask if he would consider pardoning Perry. Others on the right called for Abbott to issue a pardon, and the governor soon responded with an announcement that he would do just that, as long as the Texas Board of Pardons and Paroles recommended that Perry should be granted one.
“Trial verdicts are determined by judges and juries,” wrote Dean Obeidallah. “What Abbott is doing is not just wrong, it’s dangerous. His pardon, when it comes, is not what the rule of law looks like.”
Two of the likeliest candidates for president in 2024 haven’t officially committed yet.
President Joe Biden says he intends to run again but has delayed making a formal announcement. And Florida Gov. Ron DeSantis is making all the moves a presidential contender usually makes, including hawking his new book and visiting New Hampshire, but he hasn’t joined fellow Republicans including former President Donald Trump, former UN Ambassador Nikki Haley and former Arkansas Gov. Asa Hutchinson in declaring.
“DeSantis, who was neck and neck with the former president just a few months ago, may have lost a step or two in more recent polling. But his track record of successful governance in Florida should force GOP voters to think long and hard about what version of their party they want to put forward,” observed Patrick T. Brown.
“A third Trump presidential nomination would indicate that Republican primary voters may prefer style over substance. But if they are serious about not just making liberals mad but advancing actual policy, GOP voters should consider other names, starting with the Florida governor.”
Even without an official announcement by the president, wrote Julian Zelizer, the Biden-Harris campaign is very much under way. “By choosing to lie low while Republicans are gearing up for 2024, Biden is employing his version of what has become known as the ‘Rose Garden Strategy,’ whereby the incumbent campaigns by focusing on the business of being president and showing voters that he is the responsible figure in the race.”
“The president’s understated strategy makes room for Republicans to stoke chaos, tear each other apart and make unforced errors while he remains above the fray for as long as possible. This strategy makes the GOP the focus of the election, allowing Biden to reinforce his message from 2020: do voters want someone who will govern and act in a serious manner or do they want a circus?”
Gene Seymour: I am betting on Cousin Greg. But I am not a serious person (Spoiler alert)
Frida Ghitis: Amid fallout of Macron-Xi meeting, another world leader tries his luck
Michael Bociurkiw: How the battle for Bakhmut exposed Russia’s ‘meat-grinder’
Peggy Drexler: Sen. Dianne Feinstein’s dilemma is a reminder of this universal question
Christopher Howard: The overlooked problem with raising the retirement age for Social Security
Elliot Williams: The justice system Trump and other white-collar defendants see is different than what most accused criminals get
Phoebe Gavin: The hard lessons I learned the first time I was laid off
Meg Jacobs: ‘Air’ celebrates those who do the hard work and get rewarded
AND…
Jill Filipovic recently took a domestic flight in South Africa. “Passengers and airport staff alike were friendly and polite. The airplane seat offered enough room for both of my legs and both of my arms. We took off on time and landed early. My shoes stayed on the whole time I was at the airport.”
It was a vivid reminder of what’s possible in air travel — and of what’s usually lacking.
Take the security system: “More than 20 years after Sept. 11, 2001, only passengers who pay for the privilege can avoid removing their shoes and laptops from their bags by submitting their personal information ahead of time and undergoing background checks.”
Filipovic added, “Admittedly, I do pay — I don’t want to wait in a long security line, walk my stocking feet through a metal detector and have to un- and re-pack the MacBook I’ve carefully crammed into my carry-on. But the existence of pay-to-play shorter-line security options like Clear and TSA Pre-Check make clear that it is indeed possible to pre-screen a critical mass of passengers to avoid the morass of cranky people trying to pull on their shoes while re-packing their electronics.”