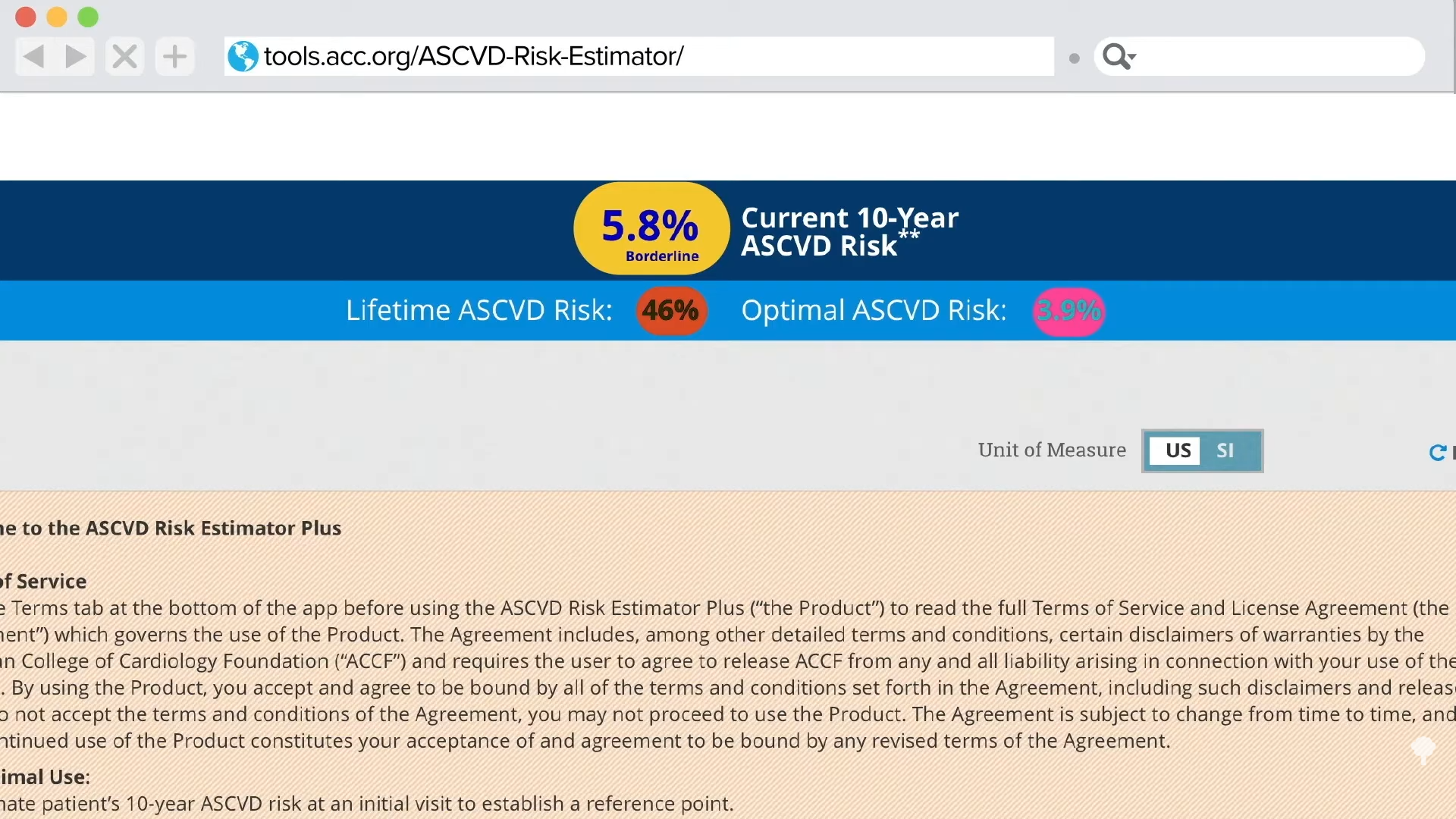
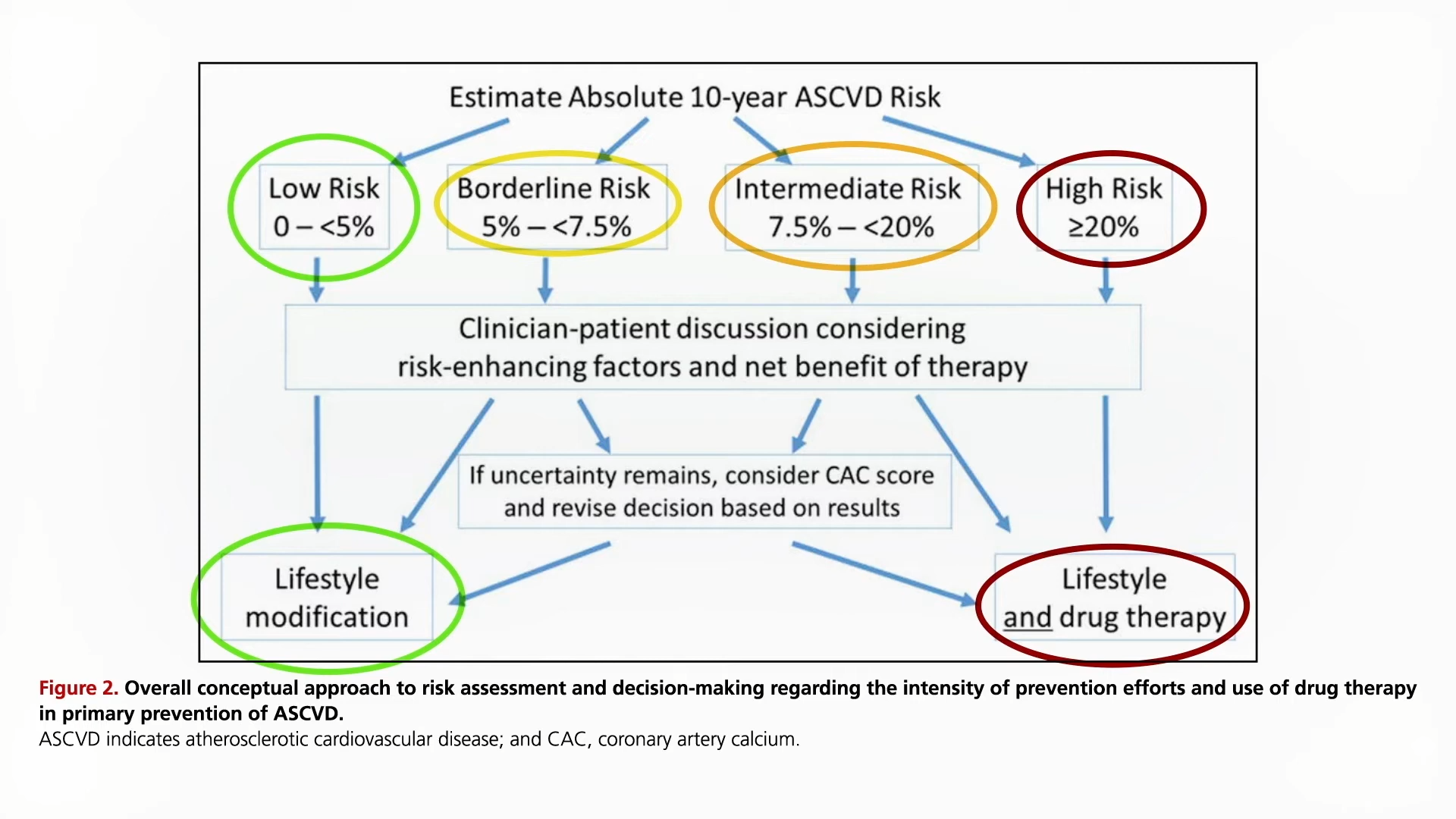
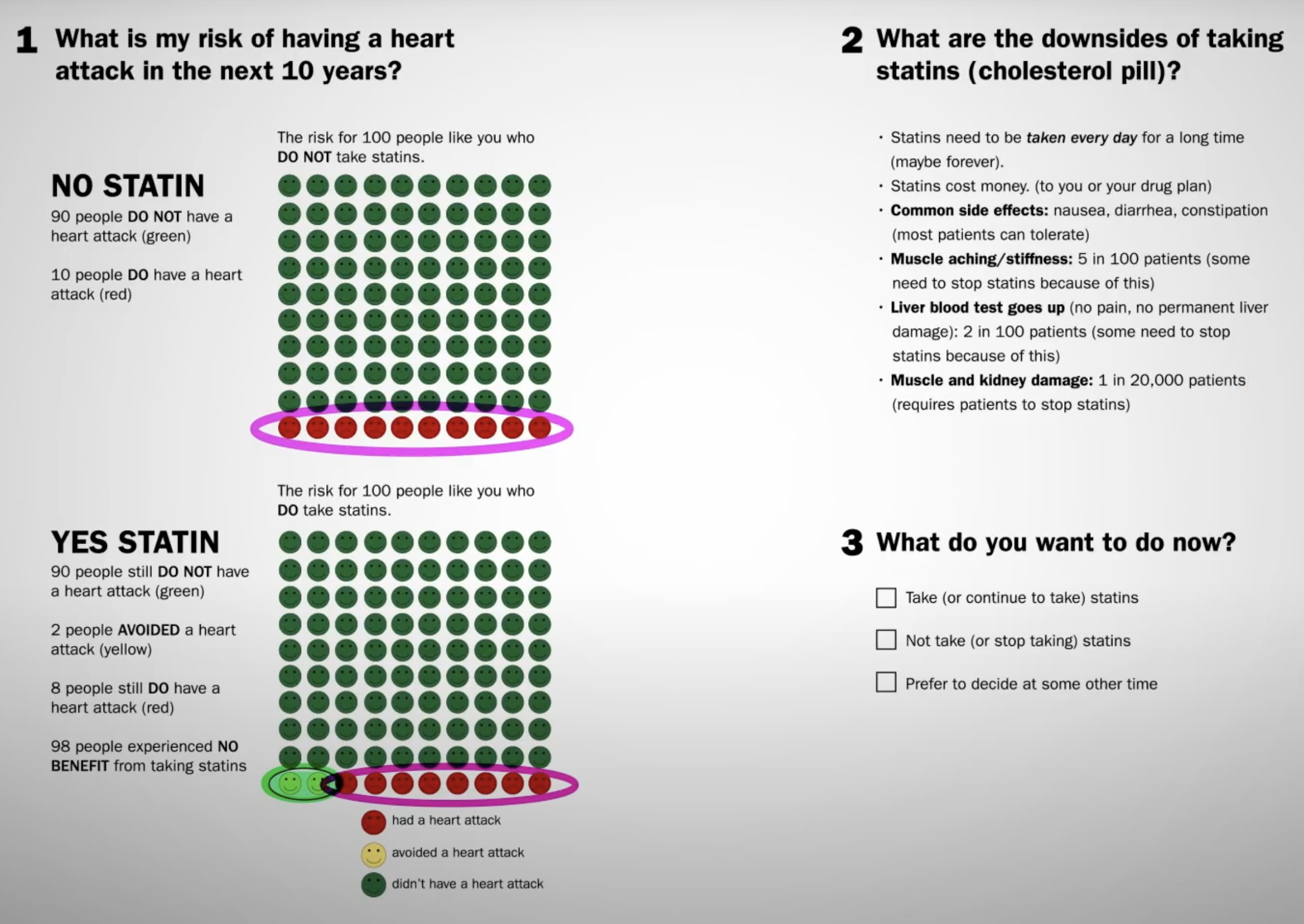
People with genetic mutations that leave them with an LDL cholesterol of 30 mg/dL live exceptionally long lives. Can we duplicate that effect with drugs?
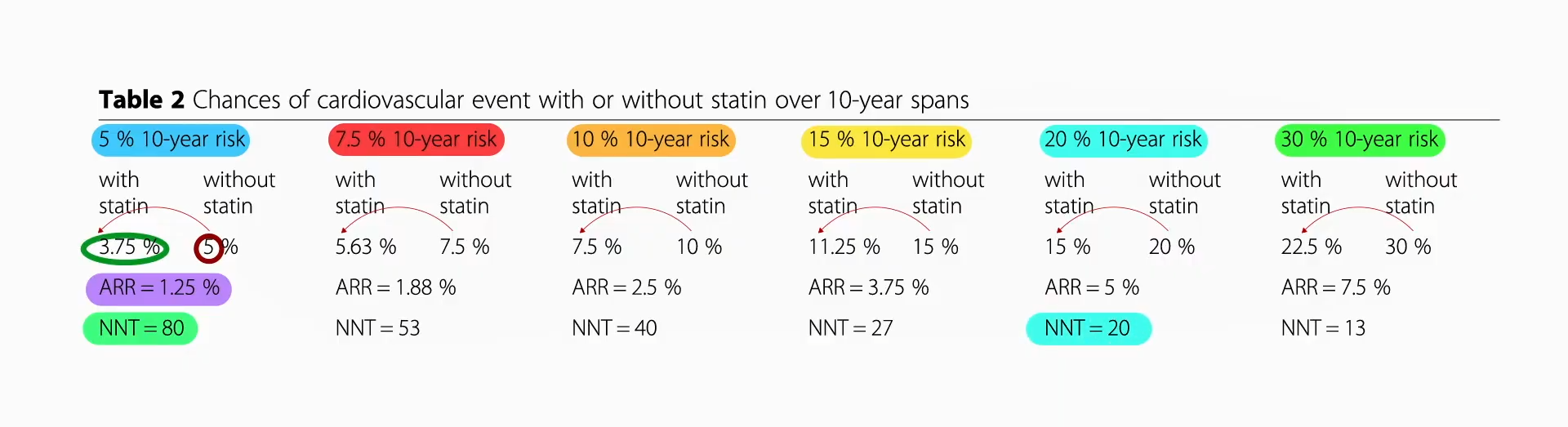
Data extrapolated from large cholesterol-lowering trials using statin drugs suggest that the incidence of cardiovascular events like heart attacks would approach zero if LDL cholesterol could be forced down below 60 mg/dL for first-time prevention and around 30 mg/dL for those trying to prevent another one. But is lower actually better? And is it even safe to have LDL cholesterol levels that low?
We didn’t know until PCSK9 inhibitors were invented. Are PCSK9 Inhibitors for LDL Cholesterol Safe and Effective? I explore that issue in my video of the same name. PCSK9 is a gene that mutated to give people such low LDL cholesterol, and that’s how Big Pharma thought of trying to cripple PCSK9 with drugs. After a heart attack, intensive lowering of an individual’s LDL cholesterol beyond a target of 70 mg/dL does seem to work better than more moderate lowering. There were fewer cardiovascular deaths, heart attacks, or strokes at an LDL less than 30 mg/dL compared with 70 mg/dL or higher, and even compared to less than 70 mg/dL. There is a consistent risk reduction even when starting as low as an average of 63 mg/dL, and pushing LDL down to 21 mg/dL, remarkably, showed “no observed offsetting” of adverse side effects.
Maybe that shouldn’t be so surprising, since that’s about the level at which we start life. And there’s another type of genetic mutation that leaves people with LDL levels of about 30 mg/dL their whole lives, and they are known to have an exceptionally long life expectancy. So, where did we get this idea that cholesterol could fall too low?
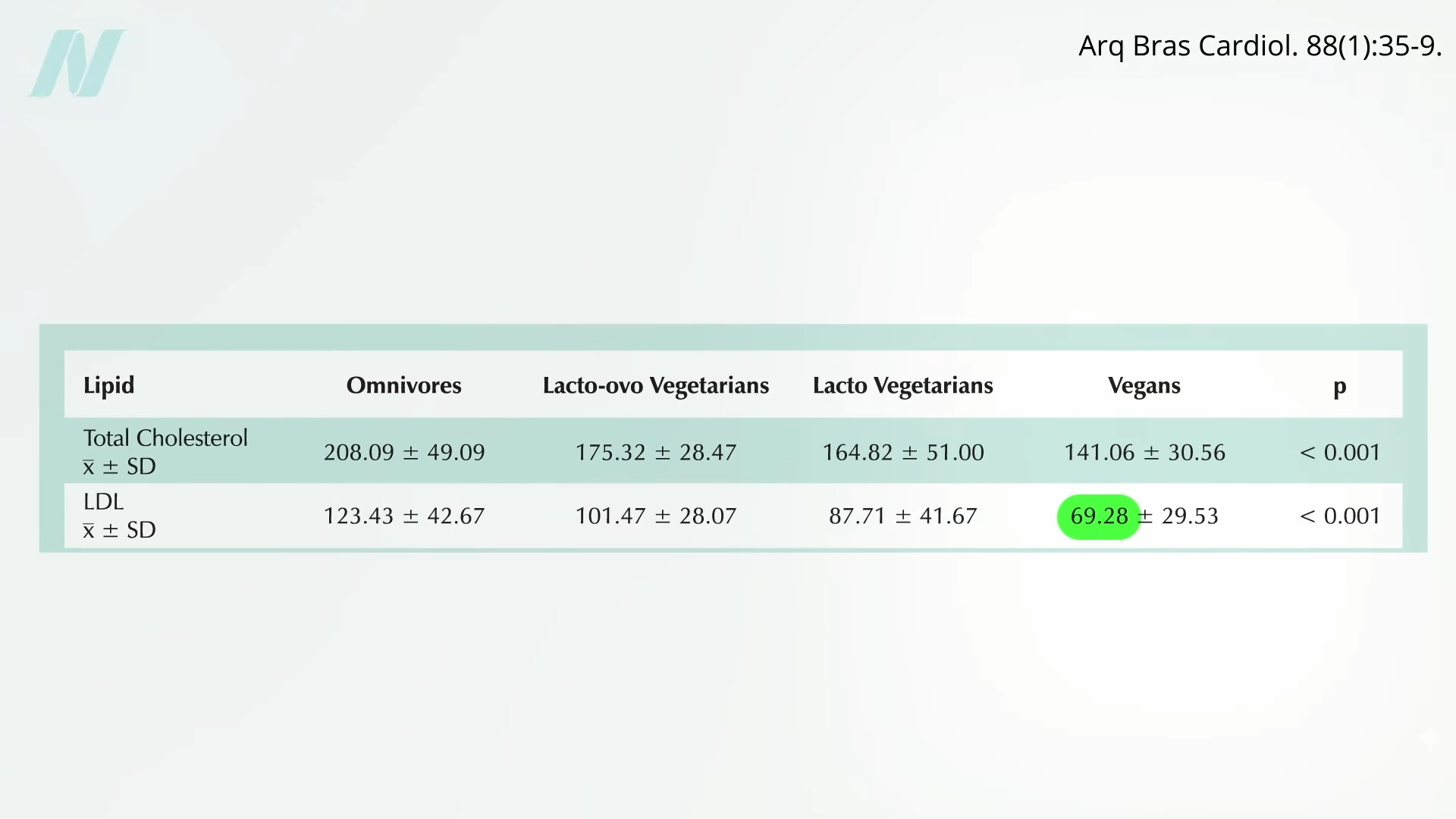
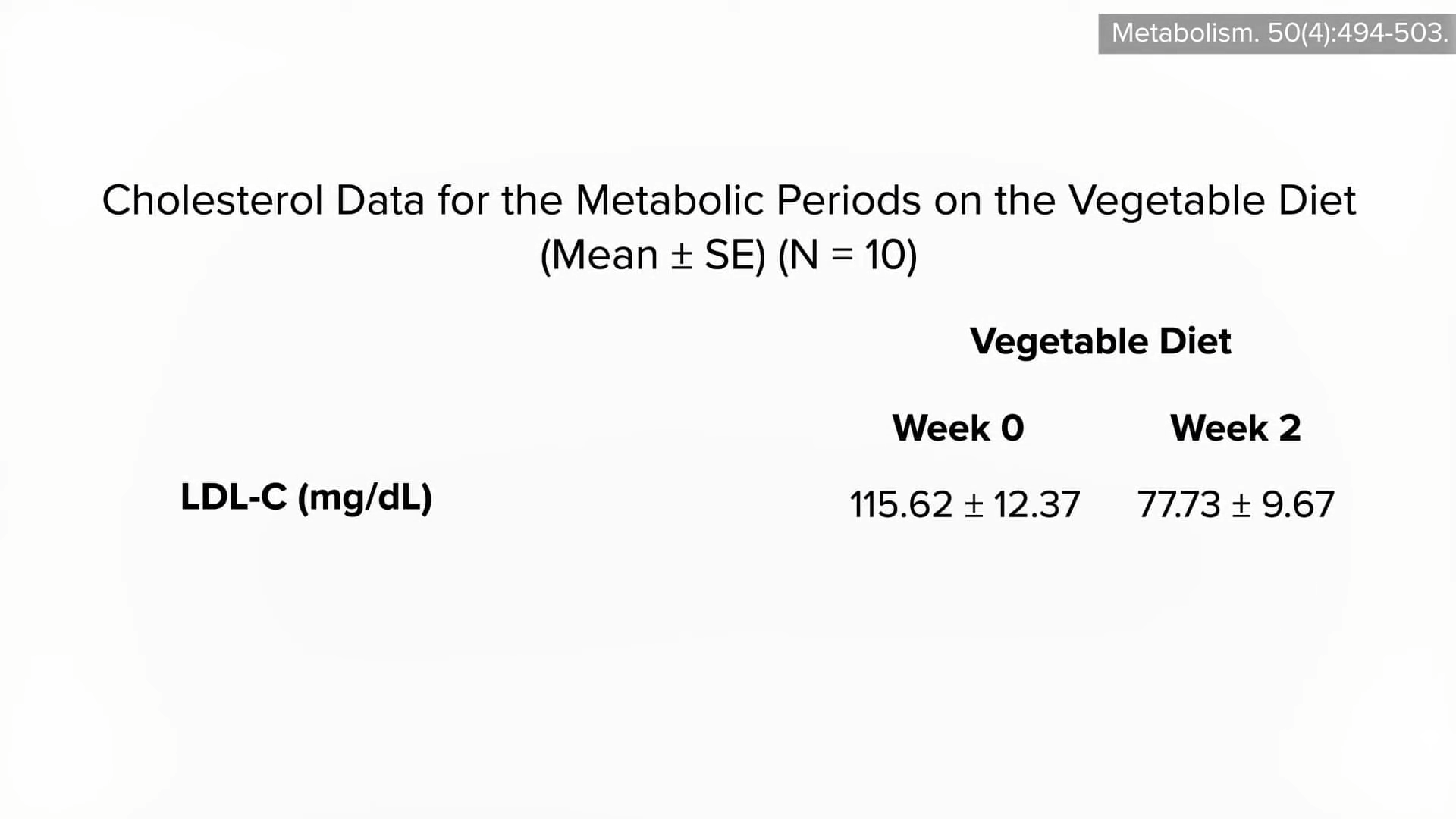
The common claim that lowering cholesterol can be dangerous due to depletion of cell cholesterol is unsupported by evidence and does not consider the exquisite balancing mechanisms our body uses. After all, that’s how we evolved. Until recently, most of us used to have LDL levels around 50 mg/dL, so that’s pretty normal for the human species. The absence of evidence that low or lowered cholesterol levels are somehow bad for us contrasts with the overwhelming evidence that cholesterol reduction decreases risk for coronary artery disease, our number one killer.
What about hormone production, though? Since the body needs cholesterol for the synthesis of steroid hormones—like adrenal hormones and sex hormones—there’s a concern that there wouldn’t be enough. You don’t know, though, until you put it to the test. For decades, we’ve known that women on cholesterol-lowering drugs don’t have a problem with estrogen production and that lowering cholesterol doesn’t affect adrenal gland function. As well, it doesn’t impair testicular function in terms of causing testosterone levels to fall below normal. If anything, statin drugs can improve erectile function in men, which is what you’d expect from lowering cholesterol. But you’ll notice these studies only looked at lowering LDL to 70 mg/dL or below. What about really low LDL?
On PCSK9 inhibitors, you can get most people under an LDL of 40 mg/dL and some under 15 mg/dL! And there is no evidence that adrenal, ovarian, or testicular hormone production is impaired, even in patients with LDL levels below 15 mg/dL. The risk of heart attacks falls in a straight line as LDL gets lower and lower, even below 10 mg/dL, for example, without apparent safety concerns, but that’s over the duration of exposure to these drugs. The longest follow-up to date of those whose LDL, by way of using multiple medications, was kept less than 30 mg/dL is six years.
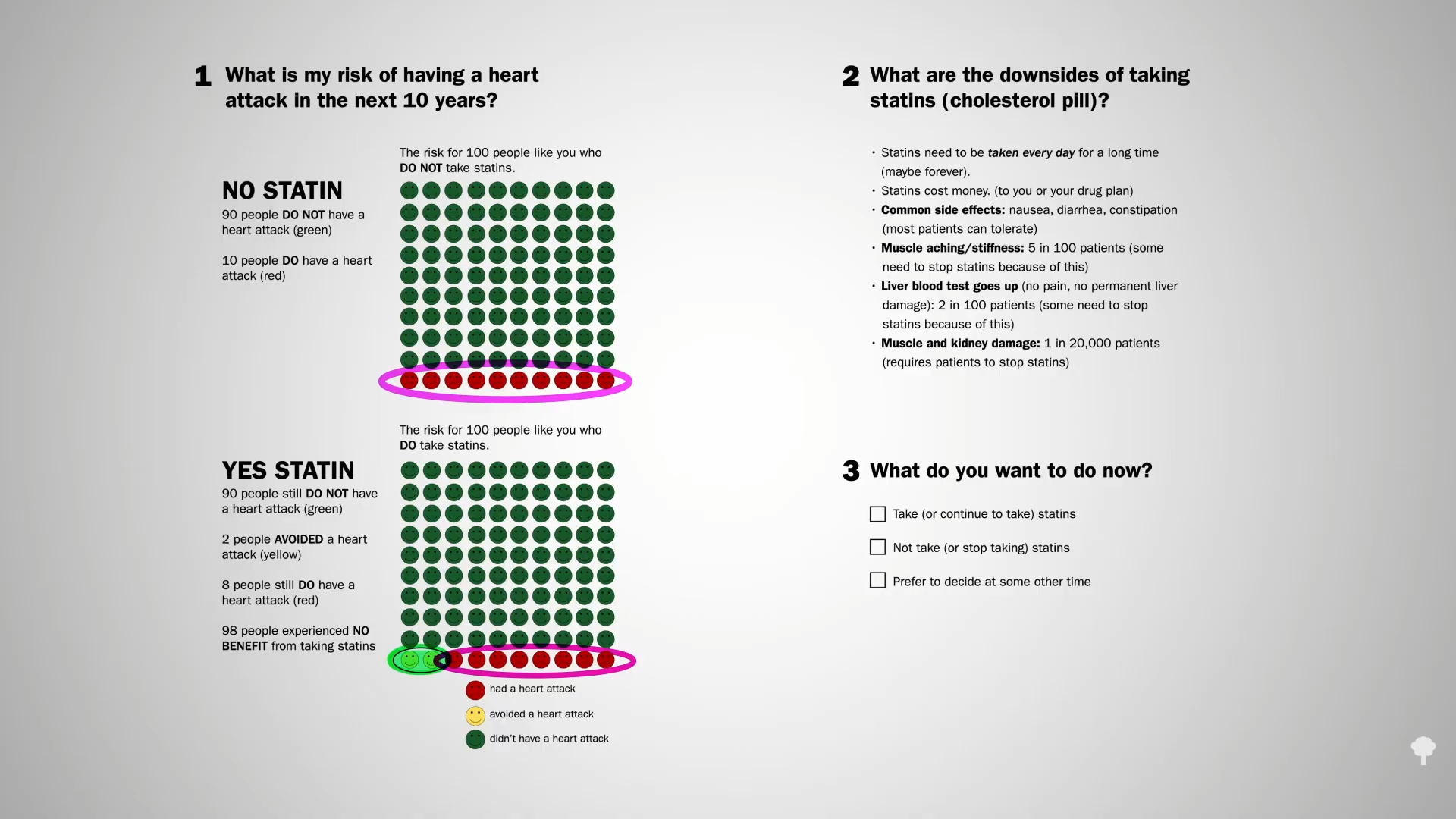
Now, we can take comfort in the fact that those with extreme PCSK9 mutations, leading to a lifelong reduction in levels of LDL to under 20 mg/dL their whole lives, remain healthy and have healthy kids. Cholesterol-affecting mutations are what cause the so-called “longevity syndromes,” but that doesn’t necessarily mean the drugs are safe. The bottom line is we should try to get our LDL cholesterol down as low as we can, but much longer follow-up data are necessary anytime a new class of drugs is introduced. So far, so good, but we’ve only been following the data for about 10 years. For example, we didn’t know statins increased diabetes risk until decades after they were approved and millions had been exposed. Also worth noting: PCSK9 inhibitors cost about $14,000 a year.
Doctor’s Note
How can we decrease cholesterol with diet? See Trans Fat, Saturated Fat, and Cholesterol: Tolerable Upper Intake of Zero.
For more on statin drugs, see the related posts below.
Michael Greger M.D. FACLM
Source link