A growing number of drugs are in short supply around the U.S., according to pharmacists.
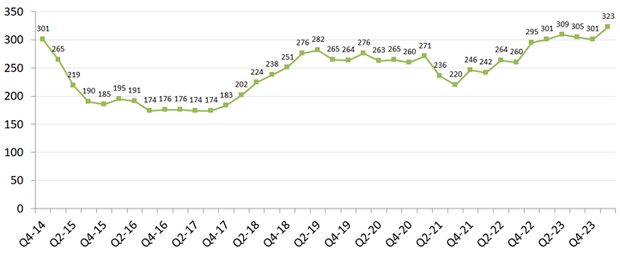
In the first three months of the year, there were 323 active medication shortages, surpassing the previous high of 320 shortages in 2014, according to a survey by the American Society of Health-System Pharmacists (ASHP) and Utah Drug Information Service. It also amounts to the most shortages since the trade group started keeping track in 2001.
“All drug classes are vulnerable to shortages. Some of the most worrying shortages involve generic sterile injectable medications, including cancer chemotherapy drugs and emergency medications stored in hospital crash carts and procedural areas,” ASHP said in a statement.
“Most of the drugs in short supply are generic, older products, and about half are injectable drugs that are hard to make,” Erin Fox, associate chief pharmacy officer, University of Utah Health, told CBS MoneyWatch. “Because the FDA says all generics are equal, the only way to compete is on price,” creating a race to the bottom that results in companies either halting production of the drugs or taking cost-saving shortcuts in quality, Fox said.
“The Biden-Harris Administration remains focused on strengthening the resilience of critical supply chains, including for medical products like pharmaceuticals,” the White House told CBS MoneyWatch on Friday. The effort to “ensure Americans can access the medicine they need when they need it,” with steps including investing $35 million in domestic production of materials needed to produce sterile injectable medicines.
Drug shortages have plagued the nation’s health care system for several decades, largely due to market failures and “misaligned incentives,” the Department of Health and Human Services stated in a paper published earlier this month.
Adderall, which is used to treat attention deficit hyperactivity disorder, is among the medications that are hardest to track down. The Drug Enforcement Administration said last fall that more than a dozen manufacturers planned to hike production of the drug, which has been in short supply since October 2022, but the problem persists, the pharmacist group found.
“Ongoing national shortages of therapies for Attention-Deficit/Hyperactivity Disorder (ADHD) also remains an issue for clinicians and patients,” it said.
Federal agencies have been tackling the shortages by offering pharmaceutical companies their version of carrots and sticks. The DEA, for example, recently announced quota increases of roughly 25% for methylphenidate, another type of ADHD medication, to help manufacturers to increase supply.
After noting that manufacturers had for years not been using their full quota allotments for amphetamine salts — which include Adderall — the FDA and DEA in August notified manufacturers that they need to produce to quota or return the ingredient so it could be used by another company.
American Society of Health-system Pharmacists
Contributing to the Adderall shortage include a spike in prescriptions during the pandemic, while a key manufacturer experienced production delays and other companies fell short on production targets.
The FDA recently approved several generic forms of a third type of ADHD medication, Vyvanse, or lisdexamfetamine, which could have a meaningful impact on supply, the White House said.
Most drug manufacturers did not disclose the factors behind the shortages, the pharmacy group noted in its quarterly findings. But experts have pointed to demand outstripping supplies, manufacturing constraints and disruptions in supply lines for raw materials.
As things currently stand, medicine labels are only required to name the company selling the product, not the product’s manufacturer — a lack of transparency Fox said the White House has joined the ASHP in advocating against.
Because manufacturers make so little on low-cost generic drugs, when there is a shortage, it’s only a problem for patients. The “companies are not facing any sort of hardship,” Fox added.
Meanwhile, insurance companies, as a matter of course, oftentimes only cover generic medications, putting a financial burden on patients unable to find a pharmacy that can fill the less-expensive version of a drug. “One recommendation is to call your insurance company, and ask for coverage for brand names,” Fox advised.