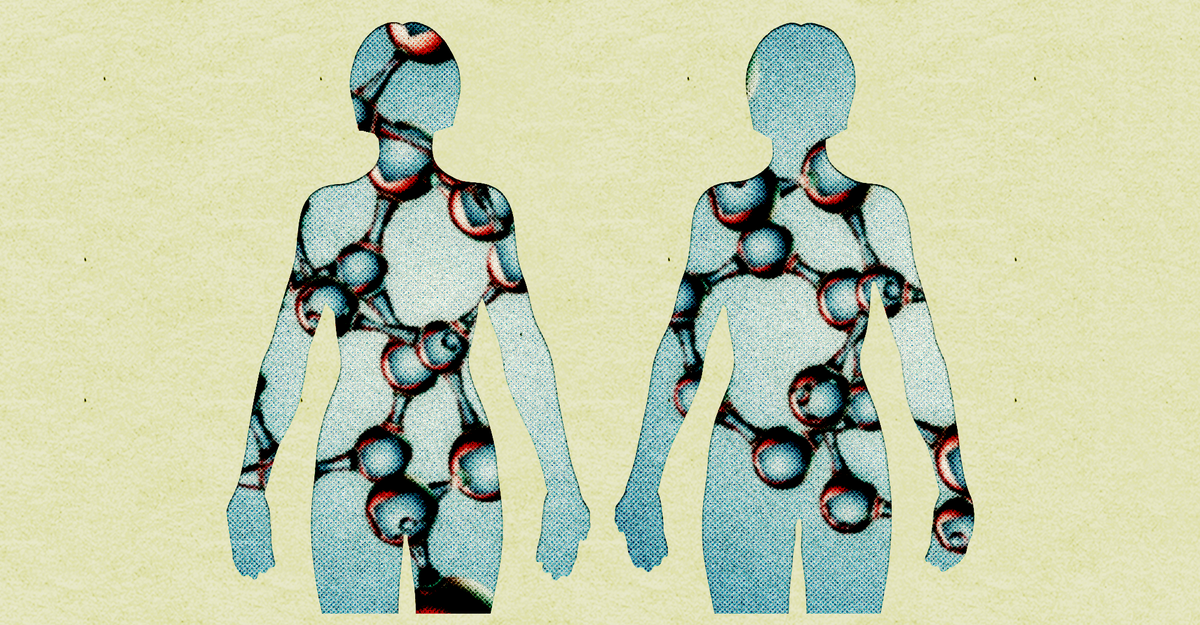
When Victoria Gray was still a baby, she started howling so inconsolably during a bath that she was rushed to the emergency room. The diagnosis was sickle-cell disease, a genetic condition that causes bouts of excruciating pain—“worse than a broken leg, worse than childbirth,” one doctor told me. Like lightning crackling in her body is how Gray, now 38, has described the pain. For most of her life, she lived in fear that it could strike at any moment, forcing her to drop everything to rush, once again, to the hospital.
After a particularly long and debilitating hospitalization in college, Gray was so weak that she had to relearn how to stand, how to use a spoon. She dropped out of school. She gave up on her dream of becoming a nurse.
Four years ago, she joined a groundbreaking clinical trial that would change her life. She became the first sickle-cell patient to be treated with the gene-editing technology CRISPR—and one of the first humans to be treated with CRISPR, period. CRISPR at that point had been hugely hyped, but had largely been used only to tinker with cells in a lab. When Gray got her experimental infusion, scientists did not know whether it would cure her disease or go terribly awry inside her. The therapy worked—better than anyone dared to hope. With her gene-edited cells, Gray now lives virtually symptom-free. Twenty-nine of 30 eligible patients in the trial went from multiple pain crises every year to zero in 12 months following treatment.
The results are so astounding that this therapy, from Vertex Pharmaceuticals and CRISPR Therapeutics, became the first CRISPR medicine ever approved, with U.K. regulators giving the green light earlier this month; the FDA appears prepared to follow suit in the next two weeks. No one yet knows the long-term effects of the therapy, but today Gray is healthy enough to work full-time and take care of her four children. “Now I’ll be there to help my daughters pick out their wedding dresses. And we’ll be able to take family vacations,” she told NPR a year after her treatment. “And they’ll have their mom every step of the way.”
The approval is a landmark for CRISPR gene editing, which was just an idea in an academic paper a little more than a decade ago—albeit one already expected to cure incurable diseases and change the world. But how, specifically? Not long after publishing her seminal research, Jennifer Doudna, who won the Nobel Prize in Chemistry with Emmanuelle Charpentier for their pioneering CRISPR work, met with a doctor on a trip to Boston. CRISPR could cure sickle-cell disease, he told her. On his computer, he scrolled through DNA sequences of cells from a sickle-cell patient that his lab had already edited with CRISPR. “That, for me, personally, was one of those watershed moments,” Doudna told me. “Okay, this is going to happen.” And now, it has happened. Gray and patients like her are living proof of gene-editing power. Sickle-cell disease is the first disease—and unlikely the last—to be transformed by CRISPR.
All of sickle-cell disease’s debilitating and ultimately deadly effects originate from a single genetic typo. A small misspelling in Gray’s DNA—an A that erroneously became a T—caused the oxygen-binding hemoglobin protein in her blood to clump together. This in turn made her red blood cells rigid, sticky, and characteristically sickle shaped, prone to obstructing blood vessels. Where oxygen cannot reach, tissue begins to die. Imagine “if you put a tourniquet on and walked away, or if you were having a heart attack all the time,” says Lewis Hsu, a pediatric hematologist at the University of Illinois at Chicago. These obstructions are immensely painful, and repeated bouts cause cumulative damage to the body, which is why people with sickle cell die some 20 years younger on average.
Not everyone with the sickle-cell mutation gets quite so sick. As far back as the 1940s, a doctor noticed that the blood of newborns with sickle-cell disease did not, surprisingly, sickle very much. Babies in the womb actually make a fetal version of the hemoglobin protein, whose higher affinity for oxygen pulls the molecule out of their mother’s blood. At birth, a gene that encodes fetal hemoglobin begins to turn off. But adults do sometimes still make varying amounts of fetal hemoglobin, and the more they make, scientists observed, the milder their sickle-cell disease, as though fetal hemoglobin had stepped in to replace the faulty adult version. Geneticists eventually figured out the exact series of switches our cells use to turn fetal hemoglobin on and off. But there, they remained stuck: They had no way to flip the switch themselves.
Then came CRISPR. The basic technology is a pair of genetic scissors that makes fairly precise cuts to DNA. CRISPR is not currently capable of fixing the A-to-T typo responsible for sickle cell, but it can be programmed to disable the switch suppressing fetal hemoglobin, turning it back on. Snip snip snip in billions of blood cells, and the result is blood that behaves like typical blood.
Sickle cell was a “very obvious” target for CRISPR from the start, says Haydar Frangoul, a hematologist at the Sarah Cannon Research Institute in Nashville, who treated Gray in the trial. Scientists already knew the genetic edits necessary to reverse the disease. Sickle cell also has the advantage of affecting blood cells, which can be selectively removed from the body and gene-edited in the controlled environment of a lab. Patients, meanwhile, receive chemotherapy to kill the blood-producing cells in their bone marrow before the CRISPR-edited ones are infused back into their body, where they slowly take root and replicate over many months.
It is a long, grueling process, akin to a bone-marrow transplant with one’s own edited cells. A bone-marrow transplant from a donor is the one way doctors can currently cure sickle-cell disease, but it comes with the challenge of finding a matched donor and the risks of an immune complication called graft-versus-host disease. Using CRISPR to edit a patient’s own cells eliminates both obstacles. (A second gene-based therapy, using a more traditional engineered-virus technique to insert a modified adult hemoglobin gene into DNA semi-randomly, is also expected to receive FDA approval for sickle-cell disease soon. It seems to be equally effective at preventing pain crises so far, but development of the CRISPR therapy took much less time.)
In another way, though, sickle-cell disease is an unexpected front-runner in the race to commercialize CRISPR. Despite being one of the most common genetic diseases in the world, it has long been overlooked because of whom it affects: Globally, the overwhelming majority of sickle-cell patients live in sub-Saharan Africa. In the U.S., about 90 percent are of African descent, a group that faces discrimination in health care. When Gray, who is Black, needed powerful painkillers, she would be dismissed as an addict seeking drugs rather than a patient in crisis—a common story among sickle-cell patients.
For decades, treatment for the disease lagged too. Sickle-cell disease has been known to Western medicine since 1910, but the first drug did not become available until 1998, points out Vence Bonham, a researcher at the National Human Genome Research Institute who studies health disparities. In 2017, Bonham began convening focus groups to ask sickle-cell patients about CRISPR. Many were hopeful, but some had misgivings because of the history of experimentation on Black people in the U.S. Gray, for her part, has said she never would have agreed to the experimental protocol had she been offered it at one of the hospitals that had treated her poorly. Several researchers told me they hoped the sickle-cell therapy would make a different kind of history: A community that has been marginalized in medicine is the first in line to benefit from CRISPR.
Doctors aren’t willing to call it an outright “cure” yet. The long-term durability and safety of gene editing are still unknown, and although the therapy virtually eliminated pain crises, Hsu says that organ damage can accumulate even without acute pain. Does gene editing prevent all that organ damage too? Vertex, the company that makes the therapy, plans to monitor patients for 15 years.
Still, the short-term impact on patients’ lives is profound. “We wouldn’t have dreamed about this even five, 10 years ago,” says Martin Steinberg, a hematologist at Boston University who also sits on the steering committee for Vertex. He thought it might ameliorate the pain crises, but to eliminate them almost entirely? It looks pretty damn close to a cure.
In the future, however, Steinberg suspects that this currently cutting-edge therapy will seem like only a “crude attempt.” The long, painful process necessary to kill unedited blood cells makes it inaccessible for patients who cannot take months out of their life to move near the limited number of transplant centers in the U.S.—and inaccessible to patients living with sickle-cell disease in developing countries. The field is already looking at techniques that can edit cells right inside the body, a milestone recently achieved in the liver during a CRISPR trial to lower cholesterol. Scientists are also developing versions of CRISPR that are more sophisticated than a pair of genetic scissors—for example, ones that can paste sequences of DNA or edit a single letter at a time. Doctors could one day correct the underlying mutation that causes sickle-cell disease directly.
Such breakthroughs would open CRISPR up to treating diseases that are out of reach today, either because we can’t get CRISPR into the necessary cells or because the edit is too complex. “I get emails now daily from families all over the world asking, ‘My son or my loved one has this disease. Can CRISPR fix it?’” says Frangoul, who has become known as the first doctor to infuse a sickle-cell patient in a CRISPR trial. The answer, usually, is not yet. But clinical trials are already under way to test CRISPR in treating cancer, diabetes, HIV, urinary tract infections, hereditary angioedema, and more. We have opened the book on CRISPR gene editing, Frangoul told me, but this is not the final chapter. We may still be writing the very first.
Sarah Zhang
Source link