This is a developing story. Stayed tune for reaction to today’s FDA decision.
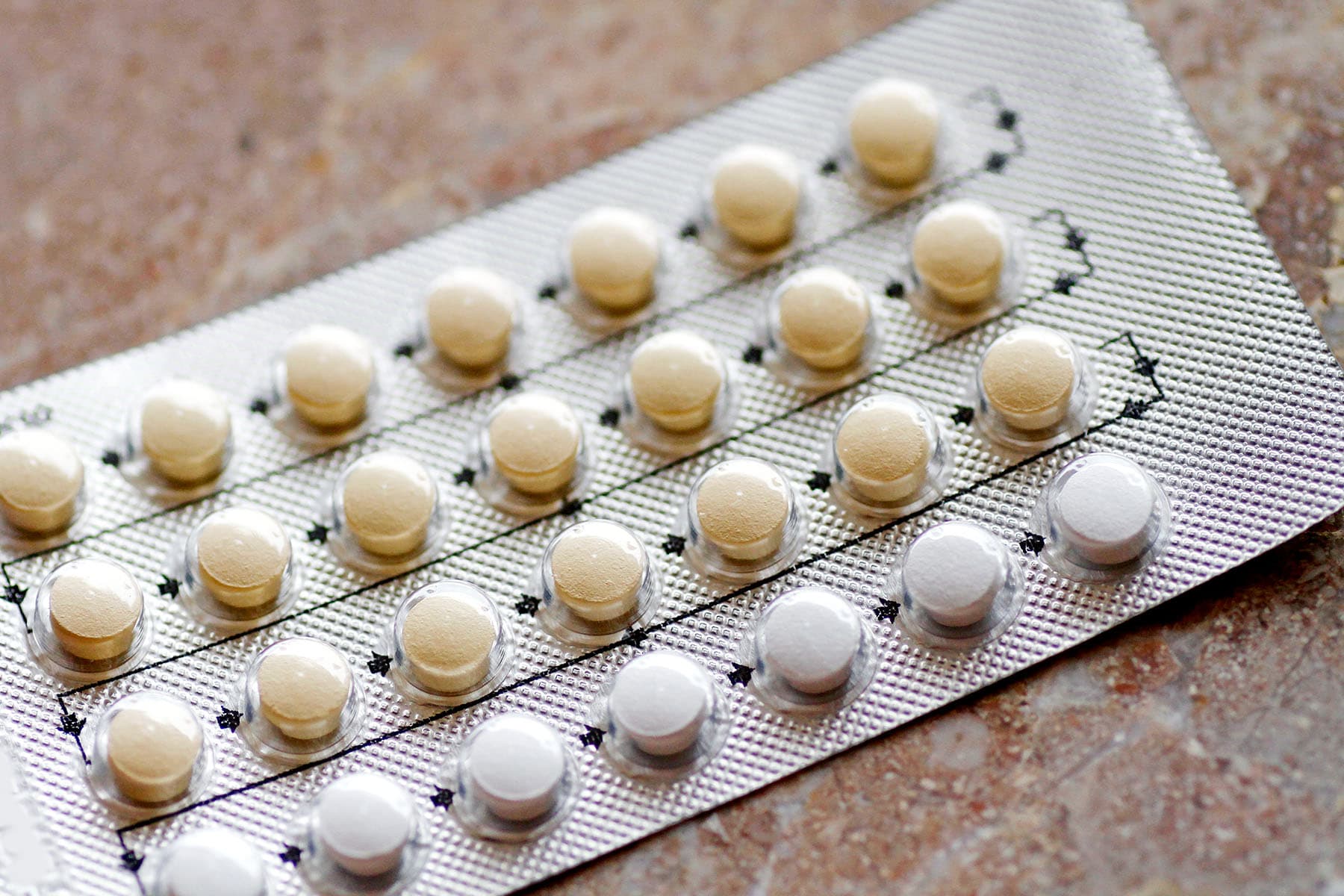
July 13, 2023 – The FDA today approved the first birth control pill for women that does not require a prescription. The product, OPill, is expected to be available early next year.
The over the counter OPill is the same norgestrel birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy or other retail location without having to see a doctor first.
The manufacturer Perrigo Company based in Ireland has not yet announced how much the pill will cost. The price tag could have implications for how widely available this form of birth control becomes. It can be as much as 93% effective in preventing pregnancy. Perrigo says it plans to make the pill available at low or no cost to some women.
The approval follows a
unanimous vote
among 17 experts on an FDA advisory committee on May 10. The panel recommended the product be made available over the counter, stating it offers more potential benefit than harm.