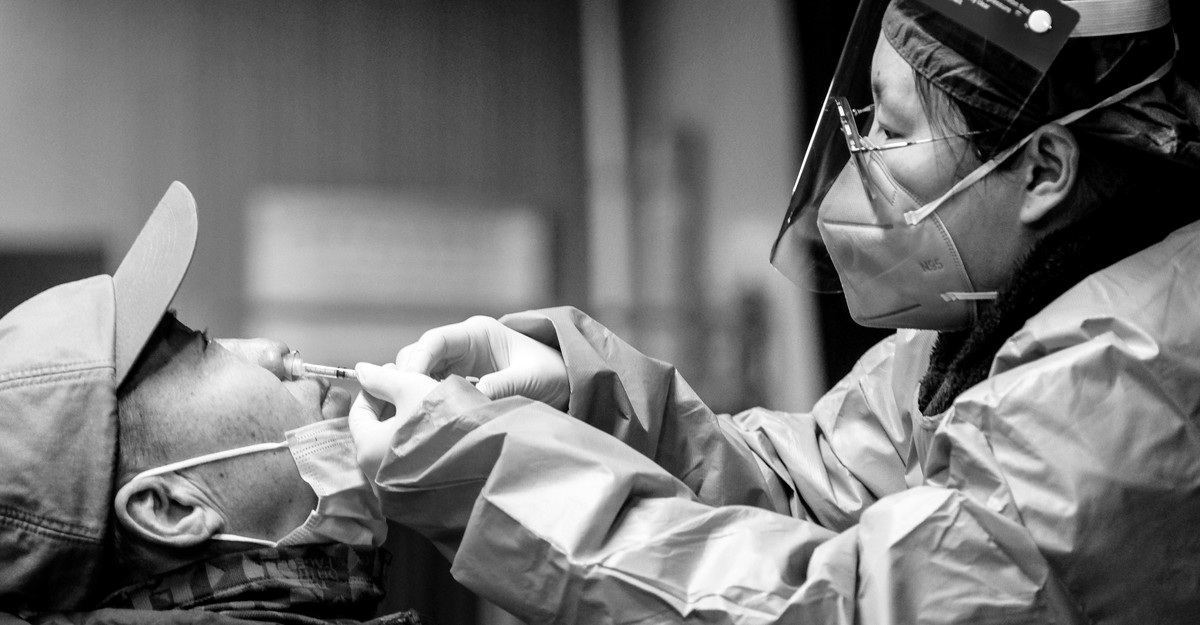
Since the early days of the coronavirus pandemic, a niche subset of experimental vaccines has offered the world a tantalizing promise: a sustained slowdown in the spread of disease. Formulated to spritz protection into the body via the nose or the mouth—the same portals of entry most accessible to the virus itself—mucosal vaccines could head SARS-CoV-2 off at the pass, stamping out infection to a degree that their injectable counterparts might never hope to achieve.
Now, nearly three years into the pandemic, mucosal vaccines are popping up all over the map. In September, India authorized one delivered as drops into the nostrils; around the same time, mainland China green-lit an inhalable immunization, and later on, a nasal-spray vaccine, now both being rolled out amid a massive case wave. Two more mucosal recipes have been quietly bopping around in Russia and Iran for many months. Some of the world’s largest and most populous countries now have access to the technology—and yet it isn’t clear how well that’s working out. “Nothing has been published; no data has been made available,” says Mike Diamond, an immunologist at Washington University in St. Louis, whose own approach to mucosal vaccines has been licensed for use in India via a company called Bharat. If mucosal vaccines are delivering on their promise, we don’t know it yet; we don’t know if they will ever deliver.
The allure of a mucosal vaccine is all about geography. Injectable shots are great at coaxing out immune defenses in the blood, where they’re able to cut down on the risk of severe disease and death. But they aren’t as good at marshaling a protective response in the upper airway, leaving an opening for the virus to still infect and transmit. When viral invaders throng the nose, blood-borne defenses have to scamper to the site of infection at a bit of a delay—it’s like stationing guards next to a bank’s central vault, only to have them rush to the entrance every time a robber trips an external alarm. Mucosal vaccines, meanwhile, would presumably be working at the door.
That same logic drives the effectiveness of the powerful oral polio vaccine, which bolsters defenses in its target virus’s preferred environment—the gut. Just one mucosal vaccine exists to combat a pathogen that enters through the nose: a nasal spray made up of weakened flu viruses, a version of which is branded as FluMist. The up-the-nose spritz is reasonably protective in kids, in some cases even outperforming its injected counterparts (though not always). But FluMist is much less potent for adults: The immunity they accumulate from a lifetime of influenza infections can wipe out the vaccine before it has time to lay down new protection. When it comes to cooking up a mucosal vaccine for a respiratory virus, “we don’t have a great template to follow,” says Deepta Bhattacharya, an immunologist at the University of Arizona.
To circumvent the FluMist problem, some researchers have instead concocted viral-vector-based vaccines—the same group of immunizations to which the Johnson & Johnson and AstraZeneca COVID shots belong. China’s two mucosal vaccines fall into this category; so does India’s nose-drop concoction, as well as a nasal version of Russia’s Sputnik V shot. Other researchers are cooking up vaccines that contain ready-made molecules of the coronavirus’s spike protein, more akin to the shot from Novavax. Among them are Iran’s mucosal COVID vaccine and a newer, still-in-development candidate from the immunologist Akiko Iwasaki and her colleagues at Yale. The Yale group is also testing an mRNA-based nasal recipe. And the company Vaxart has been tinkering with a COVID-vaccine pill that could be swallowed to provoke immune cells in the gut, which would then deploy fighters throughout the body’s mucosal surfaces, up through the nose.
Early data in animals have spurred some optimism. Trial versions of Diamond’s vaccine guarded mice, hamsters, and monkeys from the virus, in some cases seeming to stave off infection entirely; a miniaturized version of Vaxart’s oral vaccine was able to keep infected hamsters from spreading the coronavirus through the air. Iwasaki is pursuing an approach that deploys mucosal vaccines exclusively as boosters to injected shots, in the hopes that the initial jab can lay down bodywide immunity, a subset of which can then be tugged into a specialized compartment in the nose. Her nasal-protein recipe seems to trim transmission rates among rodents that have first received an in-the-muscle shot.
But attempts to re-create these results in people yielded mixed results. After an intranasal version of the AstraZeneca vaccine roused great defenses in animals, a team at Oxford moved the immunization into a small human trial—and last month, published results showing that it hardly triggered any immune response, even as a booster to an in-the-arm shot. Adam Ritchie, one of the Oxford immunologists behind the study, told me the results don’t necessarily spell disaster for other mucosal attempts, and that with more finagling, AstraZeneca’s vaccine might someday do better up the nose. Still, the results “definitely put a damper on the excitement around intranasal vaccines,” says Stephanie Langel, an immunologist at Case Western Reserve University, who’s partnering with Vaxart to develop a COVID-vaccine pill.
The mucosal COVID vaccines in India and China, at least, have reportedly shown a bit more promise in small, early human trials. Bharat’s info sheet on its nasal-drop vaccine—the Indian riff on Diamond’s recipe—says it bested another locally made vaccine, Covaxin, at tickling out antibodies, while provoking fewer side effects. China’s inhaled vaccine, too, seems to do reasonably well on the human-antibody front. But antibodies aren’t the same as true effectiveness: Vaccine makers and local health ministries, experts told me, have yet to release large-scale, real-world data showing that the vaccines substantially cut down on transmission or infection. And although some studies have hinted that nasal protection can stick around in animals for many, many months, there’s no guarantee the same will be true in humans, in whom mucosal antibodies, in particular, “are kind of known to wane pretty quickly,” Langel told me.
SARS-CoV-2 infections have offered sobering lessons of their own. The nasal immune response to the virus itself is neither impenetrable nor particularly long-lived, says David Martinez, a viral immunologist at the University of North Carolina at Chapel Hill. Even people who have been both vaccinated and infected can still get infected again, he told me, and it would be difficult for a nasal vaccine to do much better. “I don’t think mucosal vaccines are going to be the deus ex machina that some people think they’re going to be.”
Mucosal vaccines don’t need to provide a perfect blockade against infection to prove valuable. Packaged into sprays, drops, or pills, immunizations tailor-made for the mouth or the nose might make COVID vaccines easier to ship, store, and distribute en masse. “They often don’t require specialized training,” says Gregory Poland, a vaccinologist at the Mayo Clinic—a major advantage for rural or low-resource areas. The immunizing experience could also be easier for kids or anyone else who’d rather not endure a needle. Should something like Vaxart’s encapsulated vaccine work out, Langel told me, COVID vaccines could even one day be shipped via mail, in a form safe and easy enough to swallow with a glass of water at home. Some formulations may also come with far fewer side effects than, say, the mRNA-based shots, which “really kick my ass,” Bhattacharya told me. Even if mucosal vaccines weren’t a transmission-blocking knockout, “if it meant I didn’t have to get the mRNA vaccine, I would consider it.”
But the longer that countries such as the U.S. have gone without mucosal COVID vaccines, the harder it’s gotten to get one across the finish line. Transmission, in particular, is tough to study, and Langel pointed out that any new immunizations will likely have to prove that they can outperform our current crop of injected shots to secure funding, possibly even FDA approval. “It’s an uphill battle,” she told me.
Top White House advisers remain resolute that transmission-reducing tech has to be part of the next generation of COVID vaccines. Ideally, those advancements would be paired with ingredients that enhance the life span of immune responses and combat a wider swath of variants; skimp on any of them, and the U.S. might remain in repeat-vaccination purgatory for a while yet. “We need to do better on all three fronts,” Anthony Fauci, the outgoing director of the National Institute of Allergy and Infectious Diseases, told me. But packaging all that together will require another major financial investment. “We need Warp Speed 2.0,” says Shankar Musunuri, the CEO of Ocugen, the American company that has licensed Diamond’s recipe. “And so far, there is no action.” When I asked Fauci about this, he didn’t seem optimistic that this would change. “I think that they’ve reached the point where they feel, ‘We’ve given enough money to it,’” he told me. In the absence of dedicated government funds, some scientists, Iwasaki among them, have decided to spin off companies of their own. But without more public urgency and cash flow, “it could be years to decades to market,” Iwasaki told me. “And that’s if everything goes well.”
Then there’s the issue of uptake. Musunuri told me that he’s confident that the introduction of mucosal COVID vaccines in the U.S.—however long it takes to happen—will “attract all populations, including kids … people like new things.” But Rupali Limaye, a behavioral scientist at Johns Hopkins University, worries that for some, novelty will drive the exact opposite effect. The “newness” of COVID vaccines, she told me, is exactly what has prompted many to adopt an attitude of “wait and see” or even “that’s not for me.” An even newer one that jets ingredients up into the head might be met with additional reproach.
Vaccine fatigue has also set in for much of the public. In the United States, hospitalizations are once again rising, and yet less than 15 percent of people eligible for bivalent shots have gotten them. That sort of uptake is at odds with the dream of a mucosal vaccine that can drive down transmission. “It would have to be a lot of people getting vaccinated in order to have that public-health population impact,” says Ben Cowling, an epidemiologist at the University of Hong Kong. And there’s no guarantee that even a widely administered mucosal vaccine would be the population’s final dose. The pace at which we’re doling out shots is driven in part by “the virus changing so quickly,” says Ali Ellebedy, an immunologist at Washington University in St. Louis. Even a sustained encampment of antibodies in the nose could end up being a poor match for the next variant that comes along, necessitating yet another update.
The experts I spoke with worried that some members of the scientific community—even some members of the public—have begun to pin all their hopes about stopping the spread of SARS-CoV-2 on mucosal vaccines. It’s a recipe for disappointment. “People love the idea of a magic pill,” Langel told me. “But it’s just not reality.” The virus is here to stay; the goal continues to be to make that reality more survivable. “We’re trying to reduce infection and transmission, not eliminate it; that would be almost impossible,” Iwasaki told me. That’s true for any vaccine, no matter how, or where, the body first encounters it.
Katherine J. Wu
Source link